SAS in the Operating Room
By Jennifer Wagner, Ph.D.
Prism Environmental
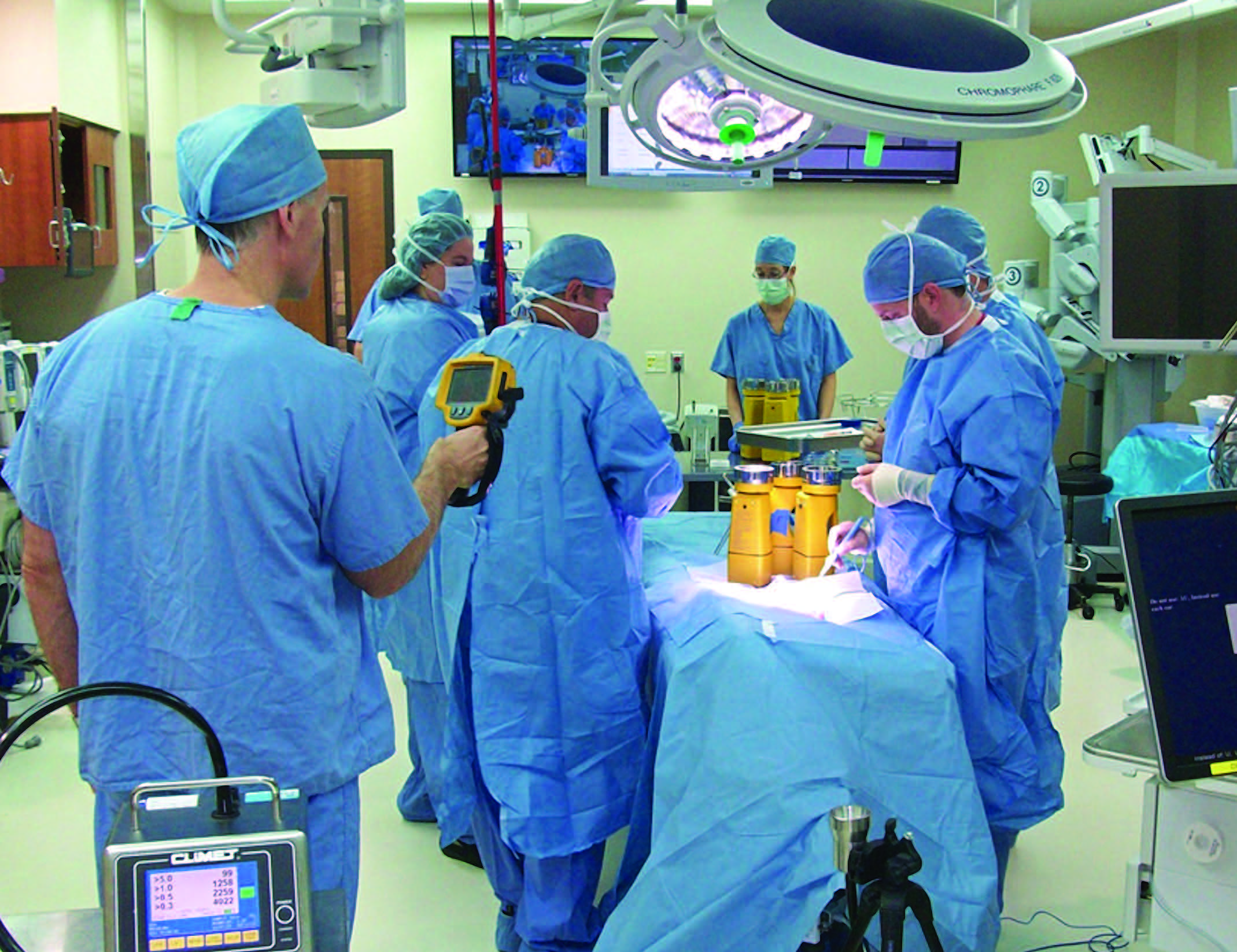
Proper ventilation of the operating room (OR) is a multifaceted challenge of OR design and engineering. The role of the HVAC system is to maintain proper air velocity, air flow patterns, humidity, temperature and pressure relationships while also assisting with asepsis. To this end, cleanrooms for compounding and microchip manufacturing have historically defined protocols for testing and data interpretation—USP <797> and ISO 14644—to ensure appropriate levels of air cleanliness, measured in either particles or microbes, that range from zero to hundreds of thousands depending on the intended application of the room. These cleanrooms also have action levels that trigger defined responses when the data exceeds a target quantifiable numerical level. These protocols are standardized across the industry and adhered to by successful manufacturers. In healthcare, however, the variation in state codes and practical application [for example, ranges in Air Change per Hour (ACH) rates from 15 to 30 ACH and higher] indicate the need to define appropriate evidence-based protocols and action levels for healthcare settings.
The interdisciplinary OnSite Environmental Quality Indicators (EQIs) Team developed and implemented protocols to measure quantifiable EQIs in a dynamic operating room environment. The process employed proven techniques and benchmarks used in pharmaceutical and semiconductor industries to measure airborne contamination in these operating rooms and compare them based on numerical classification schemes used in cleanrooms. This simulated surgical procedure and testing tool was implemented accurately at three different operating room testing sites. Meticulous detailed scripting and testing protocols yielded predictable microbial (measured with the Bioscience SAS 180 Air Sampler) and particle trends across sites, consistently classifying these ORs in a dynamic environment into numerical ISO classes extrapolated from cleanrooms. This application of employing quantifiable EQIs to OR contamination control can also be utilized in other healthcare spaces to address the efficacy of air change rate and velocity guidance, staff movement, room set-up and countless other variables. Lastly, applying these principles will assist with development of future evidence- based guidelines and codes and could help define the cost-benefit relationship of implementing these guidelines. This offers the potential to reduce operating cost and energy consumption while increasing environmental stewardship and positive outcomes.
![]()
Jennifer Wagner, Ph.D., CIC— Jennifer Wagner is founder and lead scientist at Prism Environmental and managing partner for OnSite— LLC. As co-developer and an active member of the EQI team, she dedicates her efforts to improving healthcare quality and increasing positive outcomes through evidence-based science.
Troy Markel, M.D.—Troy Markel is a pediatric surgeon in Indianapolis Indiana. As a surgeon, he is dedicated to ensuring the cleanest environments for the care of his patients. Participating in the EQI team will allow him to stay at the forefront of cleanroom methods and technology.
Thomas Gormley, Ph.D.—Tom Gormley is an Associate Professor in Construction Mgmt. at Middle Tennessee State University and adjunct at Vanderbilt University in the Mechanical Engineering Dept. His career in healthcare construction and interest in costs/benefits of air quality codes for ORs led to his Ph.D. research and mock surgical testing procedure to measure EQIs.
Damon Greeley, PE—Damon Greeley is a healthcare facilities engineering consultant based in Indianapolis, Indiana. He helps deliver reliable, effective, and efficient ventilation systems so surgical teams can perform the highest quality procedures. The EQI team provides his facilities team vital knowledge of the OR environments.
John Ostojic, IH—John Ostojic is the Sr. Industrial Hygienist for Artec Environmental based in Indianapolis, Indiana. His knowledge of indoor air quality, ventilation systems, and regulatory standards/infection control helps him to advise hospitals throughout the Midwest on both patient/employee safety and regulatory compliance.
From Bioscience World, Summer 2018
![]()
Click here for more information on the SAS air sampler.
![]()
For More Information Contact:
Bioscience International
11333 Woodglen Drive
Rockville MD 20852
Tel: 301-231-7400
Fax: 301-231-7277
Internet: BioInfo@Biosci-Intl.com
